Teaching the Trends with a 3D Teaching Aid
Who thinks that the Alexander Arrangement can't be used to teach trends should see how
we made it not only possible, but easier, faster, and better!
This is my idea:
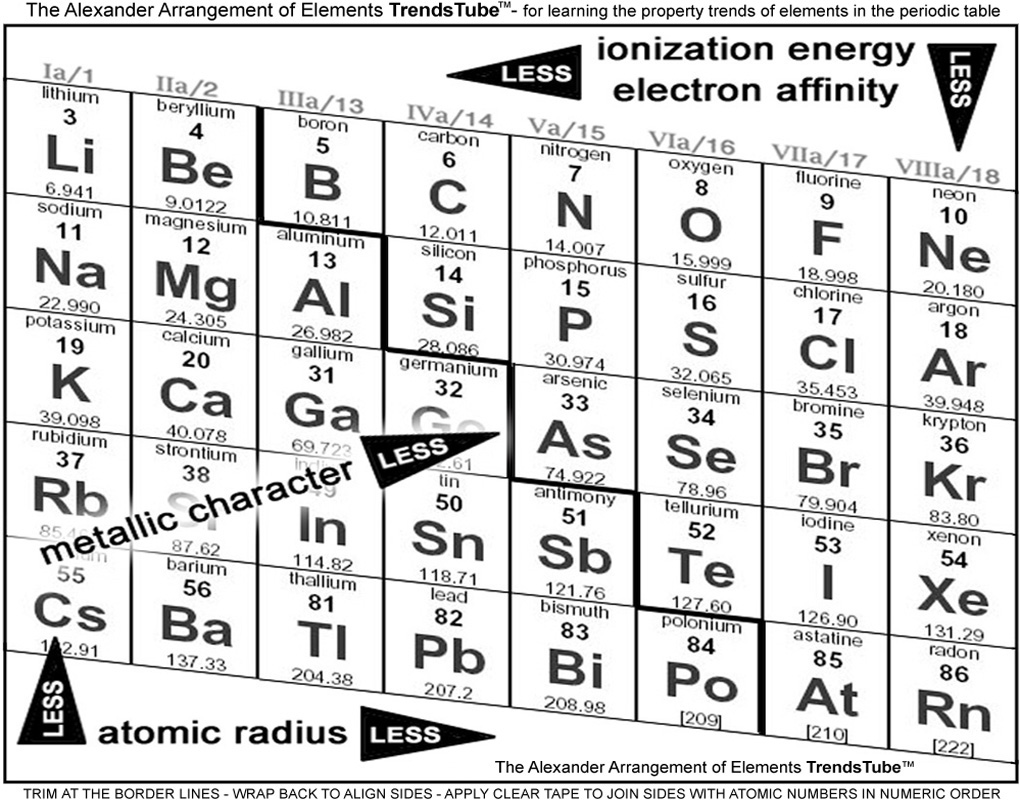
1. First, as it is obvious that not all the table was needed to make property trends clear - the rest only bring up questions and doubt - we got rid of the parts of the periodic table that don't help speed the lesson.

2. Bringing the effective parts together turns out to be the Main Group elements, the s- and p-blocks - starting at the second period.
 3. There are many properties to choose from, but we elected to use a minimum - the most frequently taught. You can petition for more, and convince us to make a second version.
3. There are many properties to choose from, but we elected to use a minimum - the most frequently taught. You can petition for more, and convince us to make a second version.

4. Other trends charts place the name of each of the trends where they are the least / smallest / weakest / etc. We couldn't understand why, so we used common logic to put the names where the properties are most / biggest / strongest / etc., and have arrows pointing in the direction of less and less.
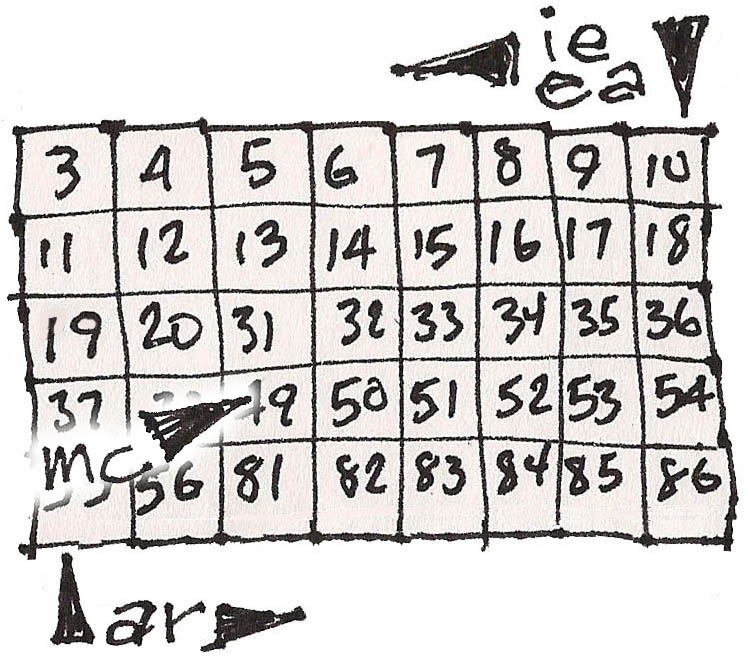
5. A full outer shell makes the Noble Gas elements independent. If the Halogens could have one more electron they would be like their next door Noble gas, and if the Alkaline metals had one less, they would be, too.
Wrapping into a tube attaches the Noble Gases to the Alkaline Metals for teaching convenience.
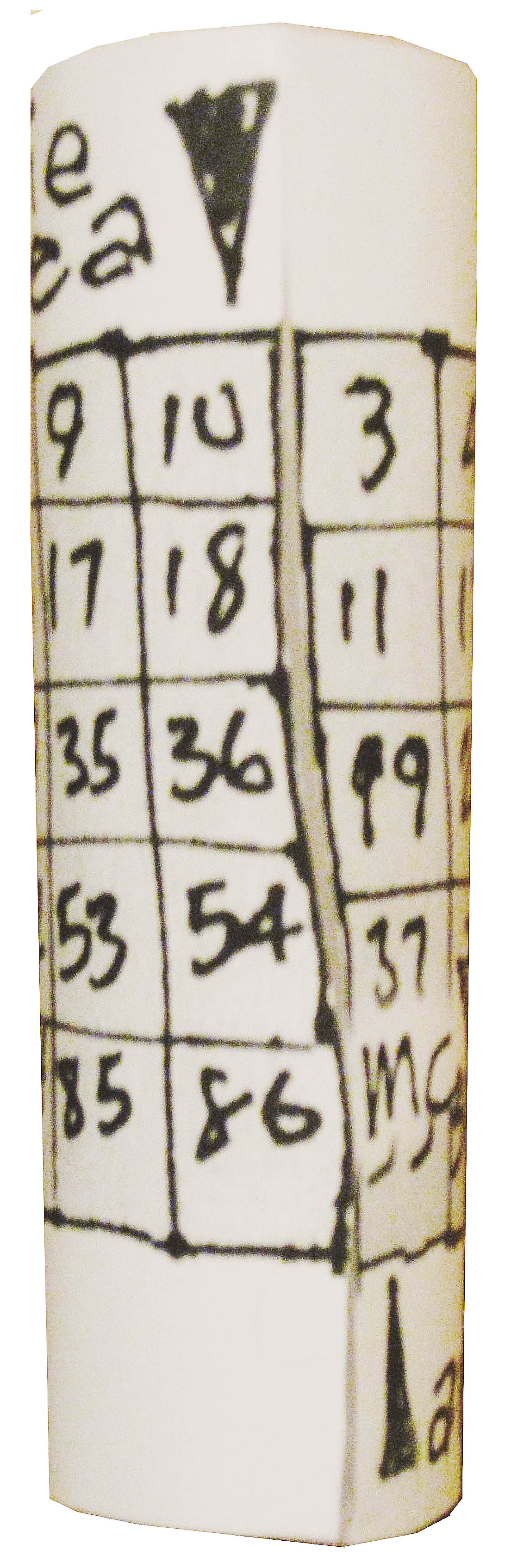
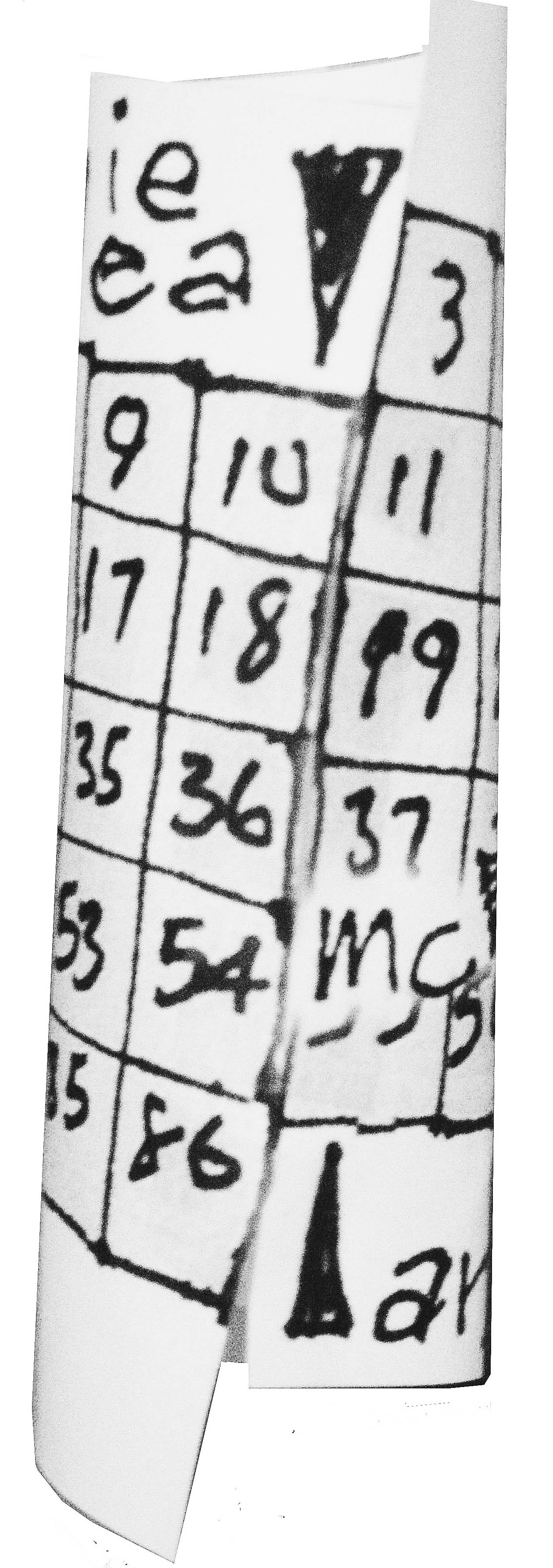
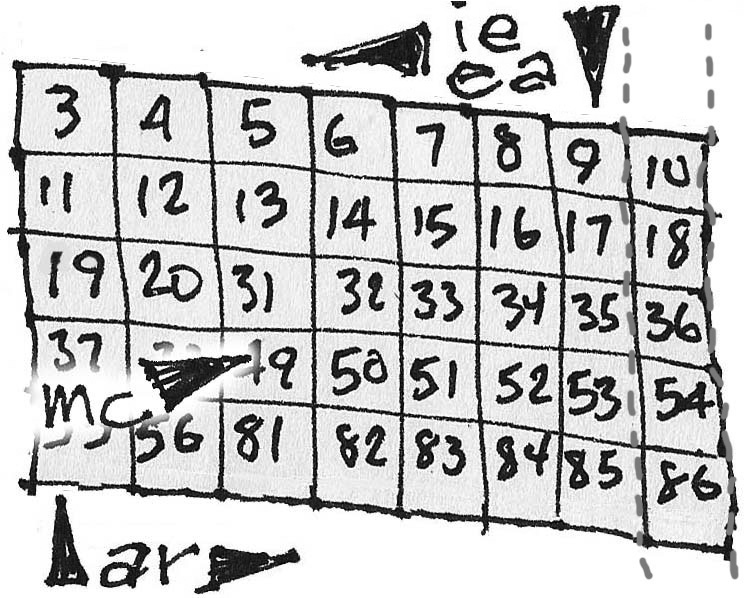
What we end up with can be seen below neatly formatted. We added a stair-step and the older US column designations (with the A suffix) to the new 1-18 as well. You can download / print / wrap it and improve your element trends lessons with it.
It just makes better sense!
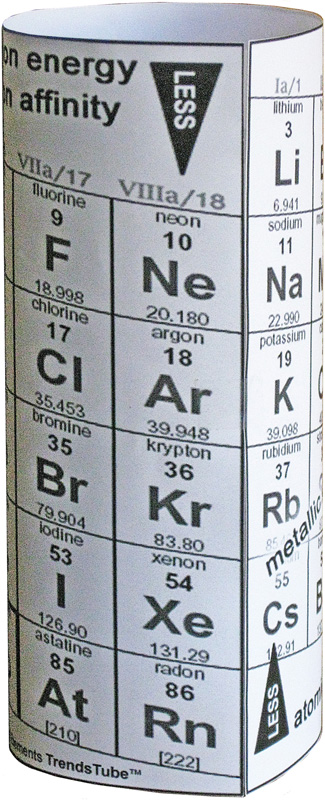
Here is the finished TrendsTube in the classroom.
< BACK
4851 N. Washtenaw Ave., Chicago, IL 60645 773.271.0318
last update 3/17/16