Alexander Arrangement of Elements Forever Periodic Table
Trends Lesson Segment
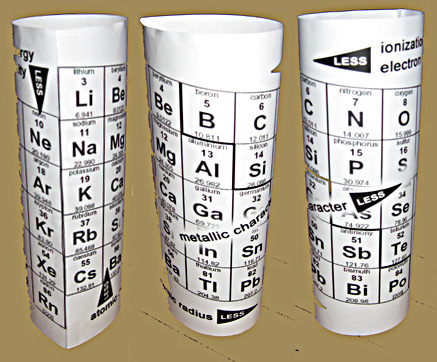
Teaching the Trends with a 3D Teaching Aid
Why switch to this chart?
Let's see if it will help you:
1. Main group elements only;
You know that the transition metals provide little help in defining trends, so why include them? Kids start out not confused enough?
To be fair, however, a number of charts DO exclude them, so it's nothing original.
2. Alkaline Metals and Halogens just an element apart;
At the other end of the familiarity scale, this is probably the most unique and best application of my Trends Chart - wrapping the ends together so the most giving and the most needy elements are just a step apart when showing the major operative factor of electron affinity.
The ordinary periodic table has them about as far apart as possible, and the picture of a teacher attempting to convince a class of skeptics that this is an important "proximity" is ludicrous. (How do YOU feel when trying to make this point - especially on a BIIIG chart?)
3. Periods connected;
The slant of our trends chart matches that of the 3D Forever Alexander Arrangement, resulting in a big help in the electron affinity aspect, a serendipitous result of making all the elements consecutive, per the Periodic Law.
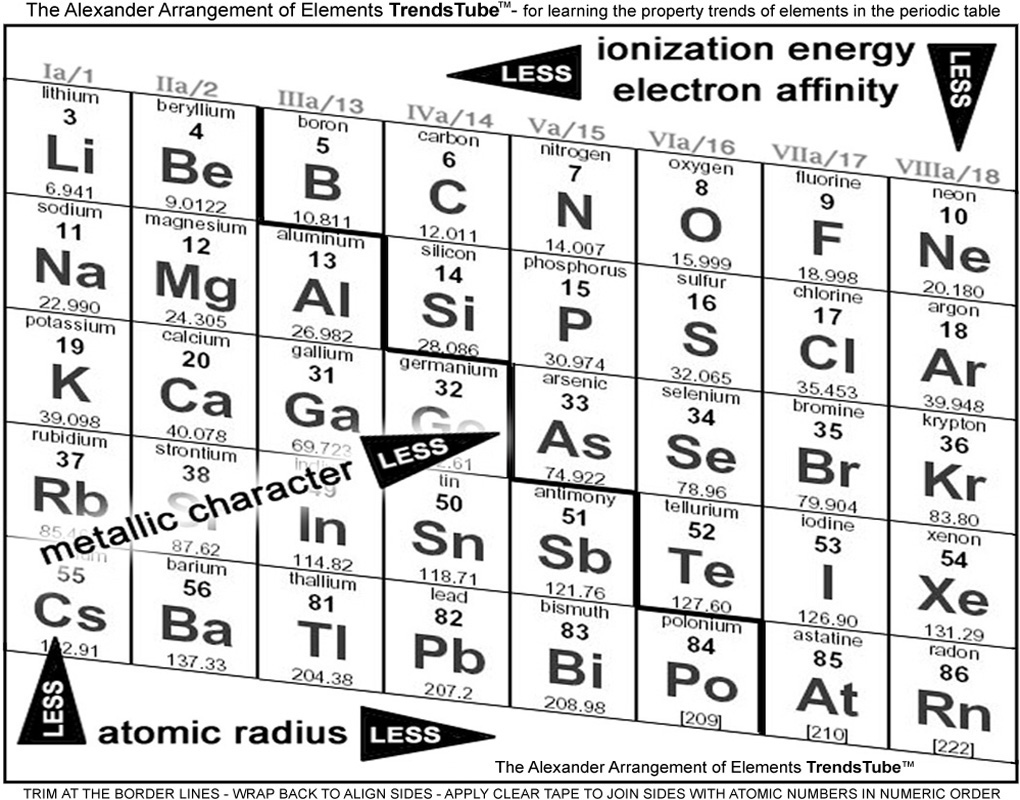
Not only are the column 17 elements and the column 1 elements just one element apart, but in that element data box is the precise element that each of them strive to become!
Eager for Nobility, they yearn to become one!
4. Trend identity positioning at the greatest;
I can't figure out why most of the charts I can find have the property name in the place where it least belongs, and arrows pointing toward the increase.
I have put the names where the property is most evident, and the arrow directs the student toward diminishing of the property ...in the ...distance.
It just seems so logical! Please tell me what I am missing here!