|
Alexander Arrangement of Elements 3D periodic table
 This model follows the principles of a three-dimensional periodic table developed by Roy Alexander: a printed representation of element information based on strict adherence to the Periodic Law, with every element data box physically and visually contiguous and continuous within the sequence of atomic numbers in generally accepted element property related columns...
This model follows the principles of a three-dimensional periodic table developed by Roy Alexander: a printed representation of element information based on strict adherence to the Periodic Law, with every element data box physically and visually contiguous and continuous within the sequence of atomic numbers in generally accepted element property related columns...
  "...the periodic table the way it's supposed to be".
"...the periodic table the way it's supposed to be".

 This is made possible by wrapping, folding, and joining what is basically a printed standard format periodic chart employing the patented p-block downslant of the element data boxes to allow the end element of a period to be adjacent to the first element of the next period.
This is made possible by wrapping, folding, and joining what is basically a printed standard format periodic chart employing the patented p-block downslant of the element data boxes to allow the end element of a period to be adjacent to the first element of the next period.
 The short periods are joined by the exit and return of the longer blocks to the same column intersection, avoiding the familiar gaps and Rare Earths displacement.
The short periods are joined by the exit and return of the longer blocks to the same column intersection, avoiding the familiar gaps and Rare Earths displacement.
Several unique features make this new Alexander Arrangement
exceptional for learning and classroom display
 The visual effect mirrors the look of Theodore Gray's series of posters, books, element cards, website, and interactive apps for the Apple iPod (requested by Steve Jobs) and iPad.
The visual effect mirrors the look of Theodore Gray's series of posters, books, element cards, website, and interactive apps for the Apple iPod (requested by Steve Jobs) and iPad.
 Each element box is dominated by a photograph of the element's simple substance or related product, with the element name, letter symbol, and atomic number often overlapping the photo.
Each element box is dominated by a photograph of the element's simple substance or related product, with the element name, letter symbol, and atomic number often overlapping the photo.
 The period numbers (below, right) are printed at the interface of the end/beginning of the periods, folded 90 degrees on the model, and the blocks and columns (old & new numbers), are identified below the data boxes - and in the case of the Actinoids, above.
The period numbers (below, right) are printed at the interface of the end/beginning of the periods, folded 90 degrees on the model, and the blocks and columns (old & new numbers), are identified below the data boxes - and in the case of the Actinoids, above.

 The element blocks connect at a central nexus (below, center), with the d- and f-blocks leaving, looping, and returning there, thus allowing the shorter period gaps above to be avoided.
The element blocks connect at a central nexus (below, center), with the d- and f-blocks leaving, looping, and returning there, thus allowing the shorter period gaps above to be avoided.
 One of the more unique teaching improvements that results from losing the gap over the d-block is the establishment of new proximity of those elements with the least and most ionization energy - on either side of the element each would like to be (see above) instead of at opposite ends of the chart.
One of the more unique teaching improvements that results from losing the gap over the d-block is the establishment of new proximity of those elements with the least and most ionization energy - on either side of the element each would like to be (see above) instead of at opposite ends of the chart.
 The p-block bends in a half-circle to join the s-block at the corner described above, with a patented 'downslant' where the element boxes gracefully sweep down a full box height (see above) within this block to allow elimination of the "carriage return" effect: each period ending on the row above the next.
The p-block bends in a half-circle to join the s-block at the corner described above, with a patented 'downslant' where the element boxes gracefully sweep down a full box height (see above) within this block to allow elimination of the "carriage return" effect: each period ending on the row above the next.

 The extended Hydrogen data box, a characteristic of all Alexander Arrangements, is even longer here, reaching all the multiple positions of the H box that are still under discussion among experts. The strip is illustrated by a hydrogen cloud in space (above, right) and loops over the s- and p-blocks. Starting behind the corner of Helium & Lithium, inside the half-helical tube to loop over Helium, attach above Lithium, Beryllium and then Carbon as the loop descends (joining the ascending portion) over the data boxes of the s- & p-blocks, ending in contact with Fluorine, Neon, and corner-on to Neon. The extended Hydrogen data box, a characteristic of all Alexander Arrangements, is even longer here, reaching all the multiple positions of the H box that are still under discussion among experts. The strip is illustrated by a hydrogen cloud in space (above, right) and loops over the s- and p-blocks. Starting behind the corner of Helium & Lithium, inside the half-helical tube to loop over Helium, attach above Lithium, Beryllium and then Carbon as the loop descends (joining the ascending portion) over the data boxes of the s- & p-blocks, ending in contact with Fluorine, Neon, and corner-on to Neon.
 Where the f-block begins and ends, between Barium and Lutetium, is the only flat segment of the element display. The pair of triangular braces that create the flat area aligns the s-block with the 'pinch' of the d-block, best seen from the bottom, when the model is hanging. (see below)
Where the f-block begins and ends, between Barium and Lutetium, is the only flat segment of the element display. The pair of triangular braces that create the flat area aligns the s-block with the 'pinch' of the d-block, best seen from the bottom, when the model is hanging. (see below)

 The model size is the largest of the production Alexander Arrangements, but for simplification and clarification of the primary educational application - introduction to property periodicity and organization of element data - the synthetic elements, those with atomic numbers over 94, are not included (see below).
The model size is the largest of the production Alexander Arrangements, but for simplification and clarification of the primary educational application - introduction to property periodicity and organization of element data - the synthetic elements, those with atomic numbers over 94, are not included (see below).
Other Parts of the Kit
Text relating to the abbreviation of the ever increasing number of elements is explained at two places on the 3D AAE illustrated periodic table model kit. One is on the model and one is separated at the time of assembly.
The statement that remains, starts under the Actinoids and continues below the d-block elements, areas where the lab created elements might ordinarily be expected to be found, says;
 The lab created elements ordinarily found in this part of a periodic table are not to be found in nature, there can be no photographs of them, so nothing needs to be added to this element photo periodic table - ever - so it will never be obsolete, a Forever Periodic Table."
The lab created elements ordinarily found in this part of a periodic table are not to be found in nature, there can be no photographs of them, so nothing needs to be added to this element photo periodic table - ever - so it will never be obsolete, a Forever Periodic Table."
The other clarification statement is;
 Naturally-occurring elements have been numbered variously, generally between 80 and 96, all for cogent scientific reasons.
Naturally-occurring elements have been numbered variously, generally between 80 and 96, all for cogent scientific reasons.
 For easier teaching and learning, we have included on this periodic table only the 92 elements actually currently existing on Earth and in the remainder of the Universe, and adding Technetium and Promethium, which, although they may have no stable forms, serve to fill what would otherwise be gaps in the sequence.
For easier teaching and learning, we have included on this periodic table only the 92 elements actually currently existing on Earth and in the remainder of the Universe, and adding Technetium and Promethium, which, although they may have no stable forms, serve to fill what would otherwise be gaps in the sequence.
 Not added for practical and educational reasons are 'elements' consisting only of pages and pages of computer data from smashing atoms in particle accelerators. Another reason is that there can be no photographs of them to show, and as a result, your arrangement is complete and never be obsolete - your Forever Periodic Table.
Not added for practical and educational reasons are 'elements' consisting only of pages and pages of computer data from smashing atoms in particle accelerators. Another reason is that there can be no photographs of them to show, and as a result, your arrangement is complete and never be obsolete - your Forever Periodic Table.
Included with the art of the periodic table on the die cut substrate that makes up the model is some background information about the the history of three dimensional periodic tables.
The first of these is about the discoverer of the concept of arranging the elements in periods suggested by the properties of the elements, de Chancourtois.
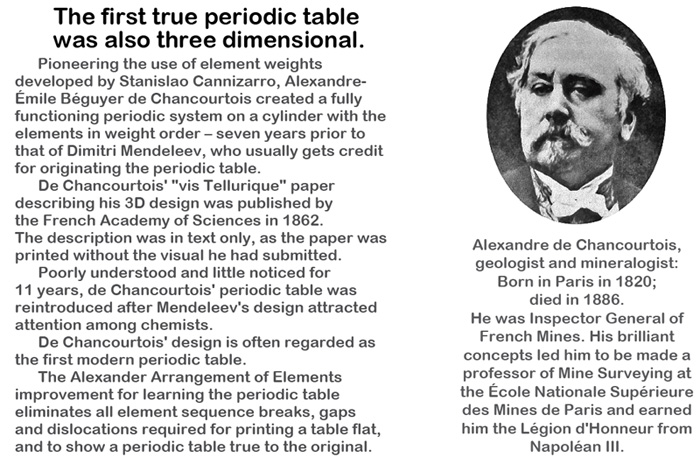
3333AA
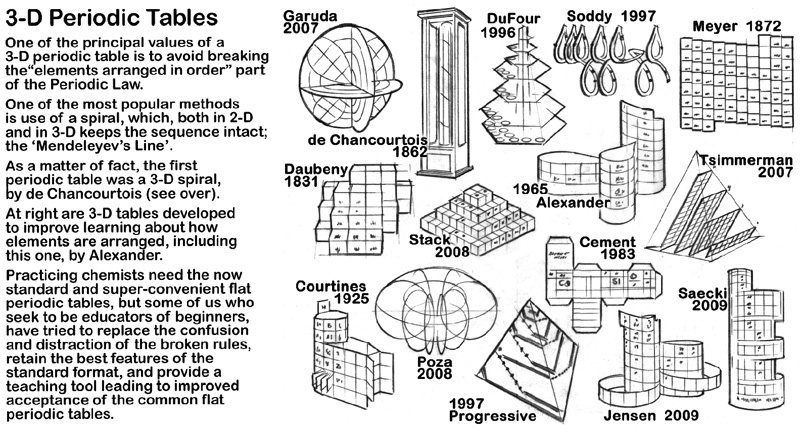
The second 3D periodic table information piece (on the flip side of the de Chancourtois
(removable card) are sketches of a variety of 3D periodic tables.
Assembly instructions and step photos were, of necessity, developed with prototype models, and have been improved and easily accessed online accompanied by an assembly video.

A number of completed model color photographs are positioned on the flip side of the instructions for easy viewing of the goal of the project.

< BACK
|







