Trends Lesson Suggestion
A 3D Aid for Teaching the Trends Easier and Better Than Ever!
Where the Halogens and Alkali Metals can be brought a Noble apart and then fully together!
Now THAT's a memorable trends lesson!
An ordinary periodic table has columns 1 and 17 about as far apart as possible,
and the attempt to convince a class of skeptics (or just smart kids)
that their's is an important "proximity" is ludicrous!
But with the story of their own personal hands-on TrendsTubes,
your students start their trends learning interactively and
with logic and clarity ...that seems like play.
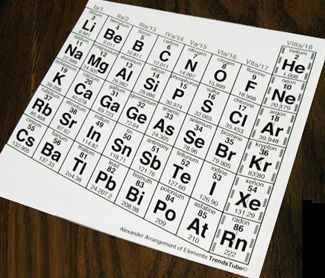
Downloaded flat prints of the TrendsTube - with instructions trimmed -
can be used within your regular introduction lesson for best directional indicators.

When the teaching gets to halogens and alkaline metals relationships,
the students can wrap and join the chart into a cylinder,
making elements of columns 1 & 17 both neighbors of the Noble Gases,
and, like their other neighbors, wanting to be like the nearest noble.

The isolation of the pretty much fully inert VIIIa group (or 18 if you prefer)
is emphasized by bending fence creases where the energetic elements to the left
and the violent of the right won't disturb the peacefullness of the
do-nothing nobility, further illustrated by gates beginning to swing shut!

When the gates are fully closed, the hard working elements 'Unionize',
finding it great to work together ...bonding - with plenty of give and take!
For free downloadable TrendsTube templates, just tap HERE!
< BACK
AlexanderDESIGN
Last Update: 4/27/20