Dmitri Mendeleev and his first published Periodic Table
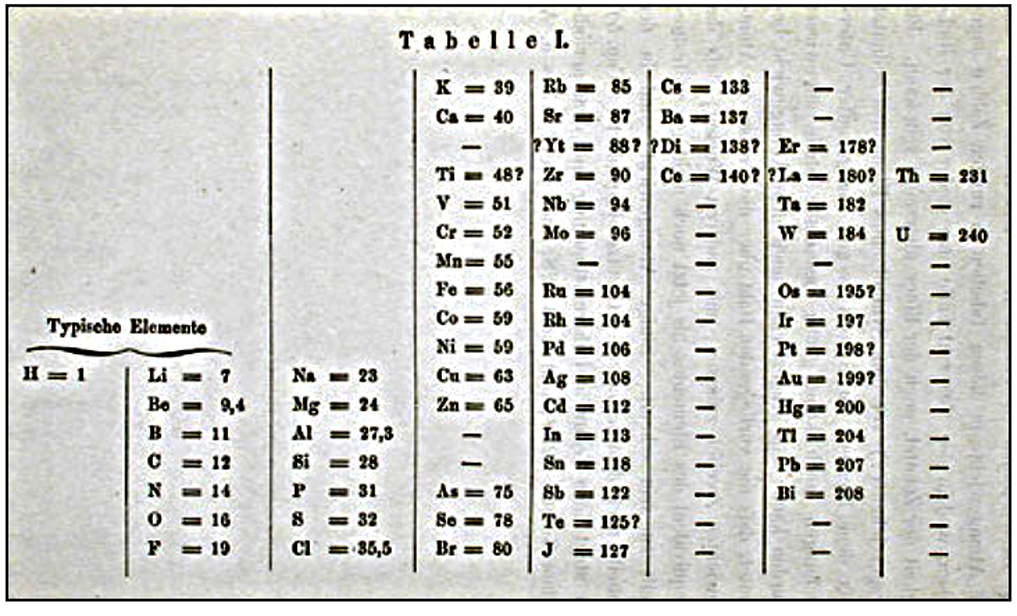 Mendeleev's original periodic table, published in 1869.
Mendeleev's original periodic table, published in 1869.
As a teacher, he was preparing a textbook for his course when he made his most important discovery. As he attempted to classify the elements according to their chemical properties, he noticed a repetitive pattern. Periodicity of elements had been previously identified by other scientists, de Chancourtois in 1862, and Meyer, but not widely known.

He was right. Three of those elements were found during his lifetime: gallium, scandium, and germanium.
The current popular flat periodic table is generally recognized as descending from his initial efforts, but it is known that he expected a three-dimensional form would be an improvement.
In1869, Mendeleev made a formal presentation to the Russian Chemical Society, titled The Dependence between the Properties of the Atomic Weights of the Elements, which described elements according to both atomic weight and valence.
Key parts of his presentation stated that:
Elements arranged according to atomic weight exhibit periodicity of properties; those with similar chemical properties either have similar atomic weights or have their atomic weights increasing regularly; the magnitude of the atomic weight determines the character of the element; discovery of many yet unknown elements must be expected - for example, two elements, analogous to aluminum and silicon, whose atomic weights would be between 65 and 75; and that certain characteristic properties of elements can be foretold from their atomic weights.
4851 N. Washtenaw Ave., Chicago, IL 60645 773.271.0318
last update 11/20/17